Chapter 10
What we found: Nutrient absorption and use
What you’ll learn in this chapter
In this chapter, we’ll look at:
- genetic variations that may affect how our bodies respond to specific diets, such as high-carbohydrate or high-fat / ketogenic diets;
- methodological problems in drawing clear links between diets and genes;
- MTHFR variants that affect folate (vitamin B9) use;
- genetic differences in caffeine metabolism; and
- what genetic testing may tell you about how your body processes particular nutrients, and what other ways you can discover this.
What role might genes play in nutrient processing?
We all know that person who seems to be able to eat “anything they want” and stay lean and fit.
Is that genetic? Possibly.
We also know that person who seems to “do everything right” and ends up with a nutrient deficiency or health problem.
Is that genetic? Possibly.
Nutrient processing (in other words, how our bodies digest, absorb, and use both macronutrients like fat, carbohydrates, and protein, along with micronutrients such as vitamins and minerals) can also be affected by many factors, such as:
- how often we exercise;
- how much body fat we have;
- the health and diversity of our gut microbiota;
- any medications we might be taking;
- etc.
In this chapter, we’ll look at what you might learn from genetic testing about the possible effects of a few dietary patterns.
Two important points to remember as you read through:
- While science is cool, and we have some interesting genetic findings and areas for further exploration, we still know comparatively very little.
- Just because a genetic test may tell you how you might metabolize a particular nutrient doesn’t mean that it can tell you the “perfect” diet or supplement for you.
As you read this chapter, remember our usual caution:
As with most preferences, health risks, and genetic traits, there are many complex, interrelated factors.
There is almost never one single gene that inevitably leads to a given result.
Any genetic data we share are simply clues for further exploration.
How do our bodies process nutrients?
When you eat an orange or some ripe blackberries that are high in vitamin C, how does that vitamin C get into your cells?
How does your body know how to extract vitamin C — or any other nutrient — from your food, absorb it, transport it where it needs to go, use it in various chemical reactions, and then get rid of any waste products it creates?
What if a nutrient needs to be in two places at once? How does your body know which one to prioritize?
Does your body prefer a certain form of a nutrient, such as the retinol form of vitamin A, instead of the plant-based carotenoid forms?
Does your body prefer to store a particular nutrient, use it immediately, or excrete it?
Does your body completely ignore an important nutrient as it sails by?
All these and many other processes and physiological decisions are governed by genetics.
Although we are more the same than different, there can be subtle genetic differences in how our unique bodies digest, absorb, use, and excrete the nutrients from the food we eat.
We can learn about some of these through genetic testing.
Macronutrients: Carbohydrates
Carbs have been getting a bad dietary rap lately. Perhaps you’ve “counted carbs” or bought a “low-carb” product… or cut them out altogether. Perhaps you’ve been told that to stay lean, carbs are the enemy. Or that needing glucose is some kind of moral failure.
As usual, science ruins our conveniently simplistic theories.
For instance, researchers studying indigenous populations living on high-carbohydrate ancestral diets full of tubers, fruit, beans, squash, honey, and heritage grains (such as maize or wild rice) reported that these traditional diets didn’t seem to make people fat or sick.
In fact, the prestigious British medical journal The Lancet recently reported on one population, the Tsimane of Bolivia, whose diet is over 70% carbohydrate from foods like manioc (cassava) and plantains, and who have some of the healthiest hearts in the world.
Such findings of metabolic health in people eating traditional diets have been consistently reported for decades.
And instead of high-carb diets inevitably making people obese (as is often suggested about processed-food Western diets), people in many other foraging societies with a traditional high-carbohydrate diet (such as the Hadza people of Tanzania) are smaller and lighter than the world average.
(By the way, the !Kung of Namibia, Botswana and Angola, and the Fulani people of northern Nigeria also have a traditionally low body mass index, or BMI, and enjoy cardiovascular health, but live on higher-fat diets. We’ll look more at potential genetic adaptations to higher-fat diets below.)
Now, of course, there are many environmental factors involved.
For instance:
- Traditional societies eat certain types of carbohydrates, generally higher-fiber, nutrient-dense types such as starchy tubers, whole grains, fruit, vegetables, and/or beans and legumes. Traditional carbohydrates are almost always lower in sugar, and digested more slowly, than modern, processed carbohydrates.
- Traditional societies are not known for their high-tech labor-saving conveniences. People would have had to work hard to get these carbohydrates (for instance, they might have to dig for tubers, or process maize or cassava so that they’re digestible).
- Traditional societies get more daily-life physical activity than people in industrialized, urbanized regions. This can affect nutrient partitioning, or how our bodies use and store those carbohydrates.
And, of course, there are genetic factors.
People who have lived a relatively traditional lifestyle for hundreds or thousands of years may also share genetic features that have made them uniquely suited to their local, ancestral diet. Over time, genetic selection helps them adapt to their environmental conditions.
Amylase and copy number variation (CNV)
In Chapter 2, we looked at the concept of copy number variation (CNV). This refers to whether chunks of genetic material are repeated. CNVs can also affect how genes work.
We used the example of genes (AMY1 and AMY2) that make an enzyme (amylase) that helps us break down the carbohydrate amylose. Digestion starts in the mouth, where salivary amylase begins the process of breaking down the starches we eat as we chew them.
Research on various populations has found that those groups with a traditionally high-carbohydrate diet (for instance, people in Japan, who consumed rice, buckwheat, sweet potatoes, and so forth) had more copies of AMY1, while people with a traditionally low-carbohydrate diet (such as people living near the Arctic Circle in places like Yakut, Russia) had fewer copies of AMY1.
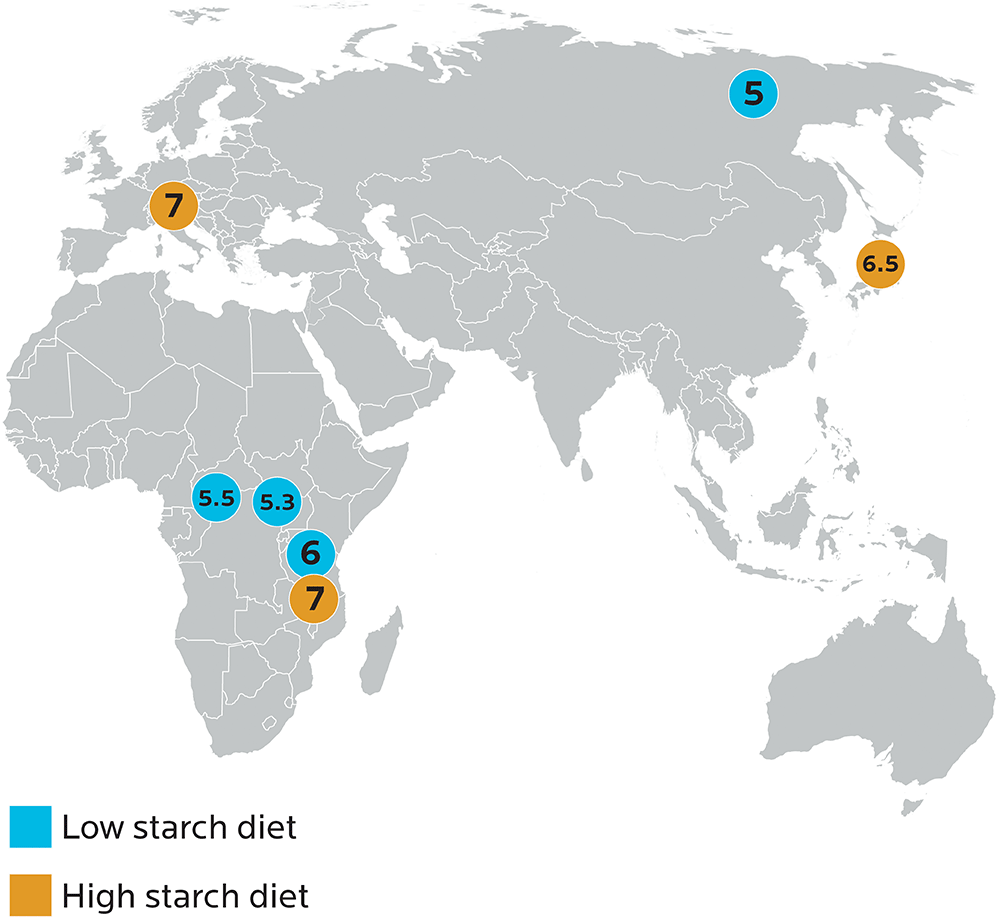
Image adapted from Perry GH, Dominy NJ, Claw KG, et al. Diet and the evolution of human amylase gene copy number variation. Nature Genetics. 2007;39(10):1256-1260.
Copy number variation of the AMY1 gene has also been linked to BMI. People who had more amylase-making genes tended to be leaner; while people with fewer copies of the genes tended to be heavier.
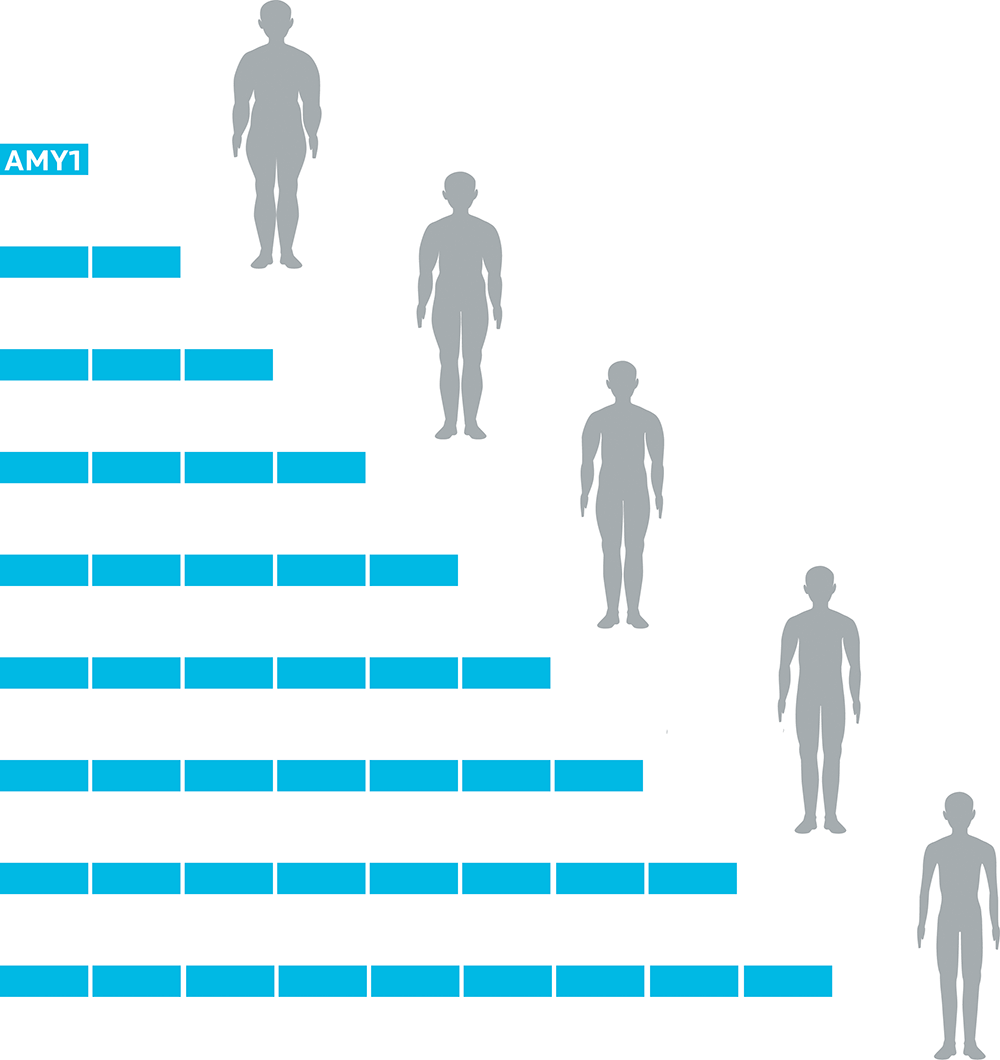
Image adapted from Falchi M, Moustafa JS, Takousis P, Pesce F, Bonnefond A, Andersson-Assarsson JC, et al. Low copy number of the salivary amylase gene predisposes to obesity. Nature genetics. 2014 May 1;46(5):492-7.
AMY1 may also play a role in postprandial (after-meal) glucose digestion and disposal. People with more copies of the gene tend to have lower blood sugar after a starch-containing meal.
In short: More copies of AMY1 might mean better starch digestion and more effective use in our bodies.
Having more AMY1 may have been a helpful adaptation for populations with high-carbohydrate diets, and it may mean that people from those populations may do well on such diets.
We don’t know for sure whether all the lean and healthy indigenous populations of the world eating high-carb diets share this genetic feature of multiple copies of amylase, or perhaps some other mechanism that helps them digest carbohydrates effectively. (For instance, a 2017 study that looked at the relationship between AMY1/2 genes and body mass index also found that AMY1A’s effects also interacted with the actions of gut microflora in digesting starches.) But it’s an interesting working hypothesis.
What this means for you
- Genetic testing may tell you about your ability to digest carbohydrates.
- Nutrigenomix tests for the rs4244372 SNP of the AMY1 gene; people with the AA variant do not digest starch as well people who are AT or TT.
- Most direct-to-consumer tests do not (yet) test for CNVs. So, we don’t have any PN sample data to share here. Many CNVs don’t have clear start and stop points (which would normally help us see where the DNA segment occurs). However, higher-end labs do have many techniques for identifying CNVs, and sequencing the exome or genome can help identify CNV.
- Genetic testing can tell you about your ancestry. As we saw in Chapter 5 with examples like lactase persistence, knowing your ancestry may suggest what types of foods or dietary patterns you might be better-adapted to eat. Research on indigenous populations who have gone back to more traditional modes of eating and living (for instance, First Nations or Inuit in Canada who have gone back to hunting, trapping, fishing, and harvesting wild foods) shows that they become healthier and fitter when following dietary patterns that better suit their evolutionary history.
- Your ancestry may also teach you about your food heritage. As we’ve seen, food isn’t just about survival or delivering nutrients; it’s about history and culture too. Learning about your genetic heritage may also inspire you to learn about your cultural food traditions and stories. This knowledge and practice is part of a “healthy diet” too.
Insulin and glucose management
Digestion of starches begins in our mouths, with salivary amylase. Another important step in carbohydrate metabolism is signaling the pancreas to release insulin.
This, too, can be affected by genetic factors.
For instance:
- The KCNJ11 gene codes for a protein that regulates insulin release. Gain-of-function mutations in this gene prevent insulin secretion in response to glucose. This gene has 219 SNPs, six of which have been linked to diabetes (rs5219, rs5215, rs5210, rs5218, rs886288, and rs2285676). 23andMe tests for the rs5219 SNP of KCNJ11. Having a T version of this allele results in a protein that doesn’t respond as well to glucose. When this happens, we can’t clear glucose as well from the bloodstream. More glucose hangs around, causing problems such as hyperglycemia, Type 2 diabetes, and tissue damage.
- We aren’t quite sure yet what the protein encoded by CDKAL1 does, but it seems to be similar to another protein that plays a role in insulin resistance. Some studies have linked SNPs in CDKAL1 (such as rs4712523) to problems with insulin secretion in European and South and East Asian populations, though other studies haven’t found the same links.
- The TCF7L2 protein, which is encoded by the TCF7L2 gene, seems to play a role in developing pancreatic islets, where we have beta cells that make insulin. If we have the T version of the rs7903146 SNP, we may make less insulin; if we are female we may also have a higher chance of developing diabetes during pregnancy (known as gestational diabetes). This link between the TCH7L2 SNP and problems with insulin has been shown in populations with European, Asian, and African ancestry.
Chapter 6 on metabolism reviews other factors that can affect glucose and insulin regulation in our bodies.
Our diverse pancreas?
The INS insulin gene can contain a variable number of tandem repeats (VNTRs), which we looked at in Chapter 5.
As per our discussion of African genetic diversity, also in Chapter 5, in African populations the INS VNTR has been divided into 22 lineages, whereas in non-African populations there are only three (grouped as class I, IIIA, and IIIB).
This particular feature is an unusually significant genetic difference between African and non-African populations.
For most polymorphisms, only 10%-15% of genetic variation is due to differences between population groups.
Yet in the case of this particular gene, estimates suggest that about 28-45% of total genetic variance is due to differences between Africans and non-Africans.
It’s not clear why this specific gene diverged so much, but it’s consistent with the hypothesis that Homo sapiens originated in Africa, and that humans who remained in Africa (while others migrated in smaller, more genetically homogeneous groups) had plenty of time to diversify.
Even more interesting, we can’t find a modern primate relative for this gene. So we can’t tell which line of the gene is the oldest.
Variation in the INS VNTR is associated with a wide range of traits and health conditions, such as Type 1 and 2 diabetes, polycystic ovarian syndrome (PCOS), birth weight, and body fat levels. This diversity may also help to explain different rates of diseases and health concerns in different populations.
What we found in our sample
23andMe tests for these genes and variants:
- CDKAL1 (rs4712523)
- CDKN2A/B (rs2383208),
- HHEX (rs1111875),
- IGF2BP2 (rs4402960),
- KCNJ11 (rs5219),
- KCNQ1 (rs2237892),
- MTNR1B (rs1387153),
- SLC30A8 (rs13266634),
- WFS1 (rs10012946), and
- PPARG (rs1801282), which we looked at in Chapter 7.
They use the above to create a combined Type 2 diabetes “risk score”.
Along with genetic data, we asked people whether they’d ever had their blood sugar tested, and if so, what it was. Not everyone had, but some were able to share.
The figure below shows the results of our multi-SNP “risk score assessment”, along with known fasting blood glucose test results.
Each row is one person. Read across to see each person’s risk score by allele, and what their actual tested blood sugar levels were (if they had been tested via blood test; if not, the space will be blank).
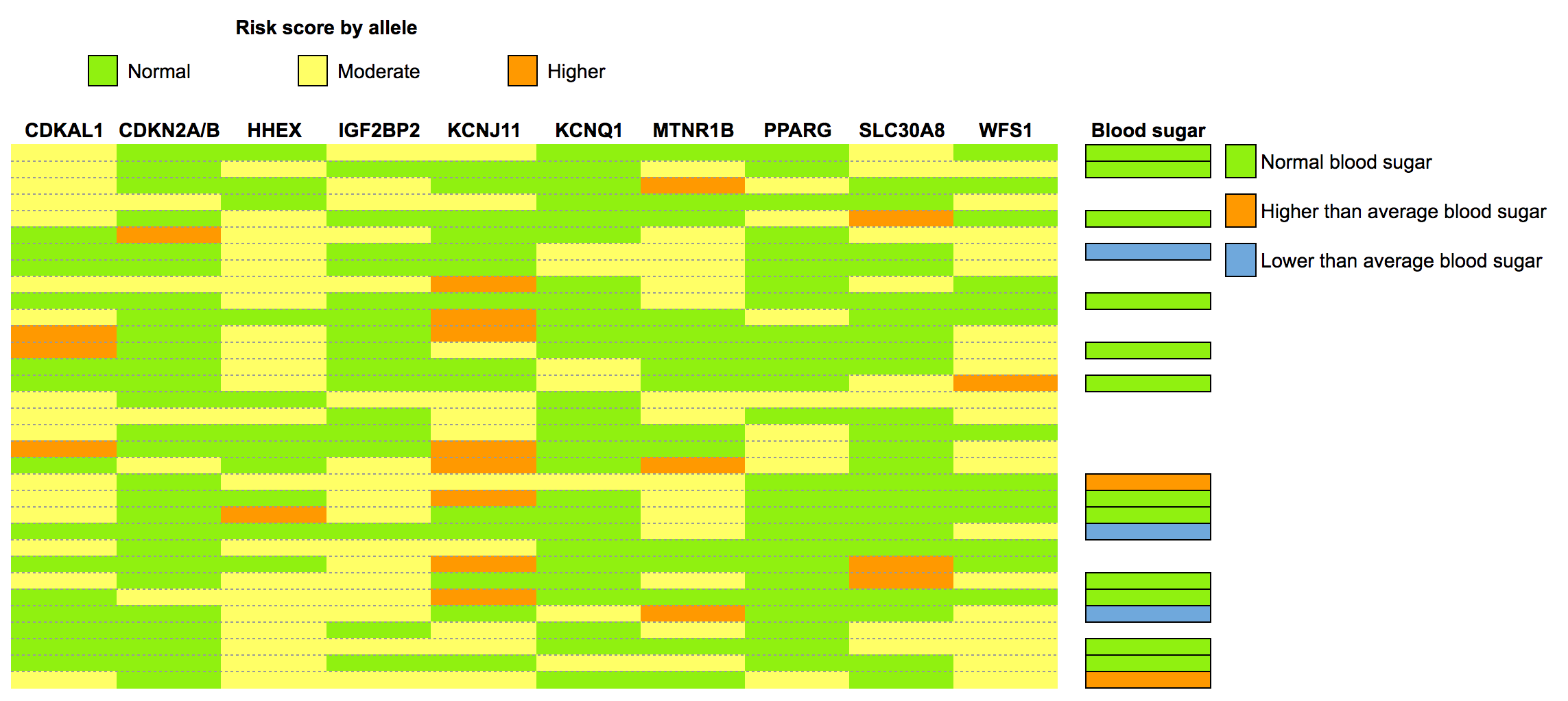
You might notice a few things here:
- Nobody had the highest possible “risk score”. Most people were, in fact, rather boringly average: a slightly higher risk here, a normal risk there.
- A few of us had lower-than-average blood sugar. One of those had a homozygous risk allele at MNTR1B.
- Of the two people who had higher-than-average blood sugar, nobody had a high-risk homozygous allele. For them, environmental factors were more relevant.
What this means for you
- Genetic testing may tell you if you have gene variants that have been linked to problems with glucose regulation. Mutations with gain or loss of function may affect how your body processes sugars and starches, as well as the function of other hormones (such as melatonin, in the case of MTNR1B) that interact with insulin.
- 23andMe tests for the following and uses them to develop a diabetes “risk score”:
- CDKAL1 (rs4712523),
- CDKN2A/B (rs2383208),
- HHEX (rs1111875),
- IGF2BP2 (rs4402960),
- KCNJ11 (rs5219),
- KCNQ1 (rs2237892),
- MTNR1B (rs1387153),
- SLC30A8 (rs13266634),
- WFS1 (rs10012946), and
- PPARG (rs1801282), which we looked at in Chapter 7.
- Again, they use these to create a combined Type 2 diabetes risk score.
- 23andMe tests for the following and uses them to develop a diabetes “risk score”:
- We probably don’t (yet) know all the genes involved. Even if you don’t have any of the “risk SNPs”, you may have others that affect your carbohydrate tolerance.
- However, other factors besides genetics can also affect your metabolic health and digestion of carbohydrates. These include:
- The type of carbohydrate you eat;
- How much you eat;
- How active you are;
- How old you are;
- Etc.
- Blood glucose is an important health indicator. Don’t rely only on a genetic test. At best, a genetic test can only predict possible risk. It can’t tell you what your blood glucose actually looks like. While blood glucose normally rises and falls as we eat and fast between meals, chronically elevated blood sugar can tell you that there is an underlying health problem.
- Get regular bloodwork done. Generally, by the time fasting glucose is affected, people may be well on their way to metabolic syndrome. So having your fasting glucose tested, along with your blood lipids, as part of your regular medical checkup (for instance, annually) is a good idea, especially as you age.
- If you are a do-it-yourself kind of person, you can buy a home glucose monitor and test your glucose throughout the day, including after meals. Postprandial — “after meals” — blood sugar levels will give you a better idea, sooner, of what your blood glucose is up to, compared to fasting glucose.
- Regardless of genetic makeup, basic nutritional principles still apply. Avoiding added sugars, minimizing processed foods, and choosing high-fiber, nutrient-rich options such as fruits and vegetables, starchy tubers, whole grains, and beans / legumes is still an excellent strategy.
- If you want to know whether your body will thrive on a higher-carb diet, just try a higher-carb diet for a month or two and see what happens. We’d define “higher-carb” as something that’s more than 50% of your total calories from carbs. Track how you feel and perform, as well as your body weight, body composition, and blood sugar if possible. The data you gather from this simple experiment will probably give you a clearer answer than most genetic predictions we could make right now.
- If you’d like to improve your overall wellness, see our strategies for healthy metabolism in Chapter 12.
Macronutrients: Fat
High-fat versus low-fat diets: Which is “healthier”? Which diet helps us stay leaner, or lose weight better? The debate has raged for decades.
One reason we can’t say definitively how much fat people should eat, or what type of fat is “best”, is because different people respond differently to different diets.
Many people are curious about whether genetic testing can answer questions like:
- How might eating a higher-fat diet change our:
- body fat / weight?
- risk of premature death?
- blood chemistry?
- How might these changes be connected to our genetic makeup, if they are?
- What specific genetic factors might be involved? (Remember that these are only associations, not causes.)
Methodological problems in studying dietary responses
Research suggests that there are, indeed, genetic differences in how people might respond to higher-fat diets.
Yet there is still no clear link between particular genes and particular outcomes.
For one thing, few studies exploring genetic responses to high-fat diets have a control group. Few are based on data where people’s food intake was strictly monitored (for instance, people getting a strict menu in a lab).
Instead, many studies about dietary intake are based on people self-reporting what they ate, which is known to be quite inaccurate.
(What did you eat for lunch last Tuesday? Tuna? What was the exact portion of tuna? Oh wait, was tuna on Wednesday? And maybe it was salmon? Difficult, right?)
So, in fact, none of the data in any study that used dietary self-reporting may be particularly useful.
This is a common problem in nutritional research in general.
Other problems in studying high-fat diets include:
- “Higher in fat” may be 30% of total calories in some studies; in other studies, it may be more.
- Fat type may differ from study to study (for instance, comparing unsaturated fats to saturated fats, or processed oils to naturally occurring fats such as butterfat).
To draw good conclusions, we need good data. So, before you get your genetic test results, recognize that the scientific research that many of these proposed genetic links are based on may have significant methodological problems.
High-fat diets and body weight
All weight loss diets need to control energy balance (energy in versus energy out). There is nothing magical about eating a given percentage of any particular macronutrient.
Yet some people swear that a high-fat diet helped them lose weight.
This may be because some people find higher-fat diets more satiating due to the effects of gastrointestinal hormones such as CCK, which respond to the presence of fat and protein in the gut. More satiating means people eat less, which then creates an energy deficit that helps them lose weight.
Other people, hearing the exciting tales of the miraculous high-fat diet, might leap on the lipid bandwagon, only to discover that, sadly, cheese and butter were not their personal path to getting ripped.
Here’s what 23andMe suggests as potential genetic markers and how they might work in European populations:
- APOA2 SNP rs5082: Associated with increased odds of obesity in those who ate a diet high in saturated fat.
- APOA5 rs662799: Having 2 copies of the G version was associated with higher BMI among those eating a diet high in fat (30% of calories from fat). The GG version of this SNP is also associated with double the risk of early heart attacks.
Other studies have looked at other links between dietary fat, other SNPs, and BMI. Results are equivocal.
For example:
- Some earlier research suggested there may be a link between the PPAR-gamma (usually written as PPAR-𝞬) pathway (which you’ll remember from Chapter 7), dietary fat, and BMI. Yet a later 2016 study involving about 11,000 European men and women found that the influence of dietary fat on associations between SNPs in the PPAR-𝞬 pathway and body size was probably “absent or marginal.”
- A study that looked at whether consumption of fried food interacted with a “genetic risk score” of 32 combined genetic variants found that yes, if people ate more fried foods they were likely to be heavier. But was this due to the greater energy intake (in other words, the fact that fried foods are high in calories), or other factors (such as other behaviors of people who eat a lot of fast food) as well? (If you’re curious, the full list of variants is here: http://www.bmj.com)
This doesn’t mean there’s no genetic link, just that we haven’t closed in on a precise connection just yet.
We have a lot of informed hypotheses and areas for further research. And we can pretty confidently say that different people have different responses to different diets.
High-fat diets and metabolic health
The rs662799 and rs662799(C) alleles of APO5 have been shown to be associated with weight gain and higher triglyceride levels on high-fat diets in a variety of ethnic groups, such as Han and Guangzhou Chinese, Pakistanis, Caribbean Hispanics, and Israelis of European (Ashkenazi), Middle Eastern (Sephardic) and Yemenite origin.
This suggests that for some people at least, high-fat diets may result in a poorer blood lipid profile.
One study in a South Korean population looked at people with a variant of the APOA5 gene that made them more likely to have high blood triglycerides. (We looked at triglycerides in Chapter 6.)
Researchers asked participants to increase their vegetable intake to six servings a day, and replace processed carbohydrates — in this case, the familiar Korean staple of white rice — with higher-fiber carbohydrate sources: whole grains (such as barley) and legumes. This higher fiber intake resulted in better blood sugar and triglycerides, and improved markers of insulin resistance (HOMA-IR), regardless of their genotype.
In other words, no matter what genes you have, eating more vegetables and fiber is a good idea for most people.
This study didn’t track weight loss. But, given what we’ve seen in our PN Coaching program, we’d guess that improving food quality and fiber intake might have loosened some people’s belts.
What we found in our sample
In our PN sample, only one person had the GG allele of APOA2 rs5082, so it was hard to draw any conclusions.
However, the APOA5 rs662799 SNP was a different story.
With this SNP, AAs were considered “normal” because they’d theoretically gain weight on a high-fat diet. GGs would be theoretically more likely to lose weight.
Nearly 80% of our sample were AAs. Some AAs did say they’d usually gain weight on a high-fat diet. This would be expected, since high-fat diets are often also high in calories. Other AAs said they’d just stay the same.
A few people did, however, report that they typically lost weight on a high-fat diet. All were AGs, not GGs.
We had no GGs in our sample. The potential rarity of this allele may help explain why some people in the fitness industry sing the praises of high-fat diets for weight loss, but also why many other people find that their personal reality doesn’t match the promise.
Of course, again, there are many other factors involved, such as:
- the energy density of fat: it packs a calorie punch and is easy to over-eat;
- people’s individual preferences for eating more fat, which may also be affected by genetics (see Chapter 8 for more on fat taste preference);
- why people might be eating a high-fat diet in the first place, since some of our PN population might be looking to gain weight on purpose; and
- how people are defining and measuring a “high-fat diet”; or
- how they were tracking their intake and observing the results.
So, we can’t really say with certainty that the APOA2 or APOA5 variants really made that much of a difference.
What this means for you
- Genetic testing may tell you whether you might gain or lose weight on a high-fat diet, or whether a high-fat diet might negatively affect your blood lipids. But right now, the research still isn’t definitive.
- You may need to watch your total fat intake — how much fat you eat in general.
- You may need to watch your saturated fat intake.
- You may gain weight on a high-fat diet. But energy balance (calories in vs. calories out) is still the most important factor. This doesn’t mean that if you have the “wrong” genes, you’re doomed to never enjoy butter or bacon again. You might simply have to moderate your intake, stay active, and make generally healthy choices… you know, all the boring non-magical stuff.
- Other dietary factors are probably also important for managing triglycerides, such as getting enough fruits and vegetables, as well as fiber from whole grains and beans / legumes.
- If you want to know whether your body will thrive on a higher-fat diet… try a higher-fat diet for a month or two, and see what happens. We’d define “higher-fat” as something that’s more than about 35-40% of your total calories from fat. Track how you feel and perform, along with your body weight, body composition, and blood lipids if possible. The data you gather from this simple experiment will probably give you a clearer answer than most genetic predictions we could make right now.
- Include a blood lipid profile as part of your regular medical checkups (e.g., annually or biannually). Again, this will give you a much clearer picture of what is actually happening right now, rather than simply speculating based on genetic markers without clear data.
Ketogenic diets
High-fat diets are often discussed together with terms like “ketosis” and “ketogenic diet”. A high-fat diet is not necessarily a ketogenic diet.
However, given that people commonly equate them, and that some form of ketogenic diet regularly comes in and out of fashion, we thought we’d discuss ketogenic diets here as well.
Ketone bodies are particular types of molecules (acetoacetate, beta-hydroxybutyrate, and acetone) that our liver can make from fatty acids. We can use these for energy, especially at times when other sources of energy aren’t readily available, such as when we’ve gone without food for a while (when fasting, most people go into ketosis within 2-3 days).
Dietary ketosis simply means that blood levels of ketones are above a certain level. Again, this most often happens when we are fasting, or when we are eating a very low carbohydrate diet (which tends to be a high-fat diet, hence the confusion between high-fat, low-carb, and ketogenic diets).
We can also raise our blood levels of ketones without changing our diets by taking ketone supplements, though this isn’t necessarily the same physiological state as ketosis via fasting or carbohydrate restriction. (Here’s a link to more reading about ketogenic diets.)
A quick skim of diet support groups will reveal that ketogenic dieting has its raving fans — people who tried ketosis-stimulating ways of eating, felt terrific, and now want everyone else to do it.
However, as with most other ways of eating, variation in key genes involved in certain metabolic pathways may shape how we respond to this or any other diet.
FGF21 and HMGCS2
You might remember FGF21 from Chapter 8 on food preferences. The FGF21 gene codes for FGF21, a protein that helps to regulate energy balance and insulin sensitivity.
Early work in mice found that the FGF21 protein increased in the liver in response to one of three states: fasting, ketogenic diets, or low-sugar / low-protein diets. This increases fat oxidation, decreases inflammation, increases energy expenditure and increases glucose tolerance. (Generally, all good things.)
However, in humans, FGF21 is only stimulated by fasting for more than 72 hours, not with other diets.
Lab models suggest that FGF21, along with other genetic and epigenetic factors, may also affect our response to ketogenic (low-carbohydrate / high-fat) diets.
The models show that:
- FGF21 protein production is turned on by ketone bodies (namely, acetoacetate) through epigenetic remodeling by a protein called SIRT3.
- This then increases the production of a protein called HMGCS2 (3-hydroxy-3-methylglutaryl-CoA synthase 2, in case you need to win biology trivia night). HMGCS2 is the first enzyme in a chain of enzymes needed to make ketone bodies.
- Ketone bodies make more FGF21 and HMGCS2.
- HMGCS2 then is able to make more ketone bodies, unless there’s a polymorphism in the HMGCS2 gene (rs28937320). There are several types of polymorphisms, one of which can be completely inactive. In that case, a ketogenic diet wouldn’t trigger FGF21, as little to no ketone bodies will be made.
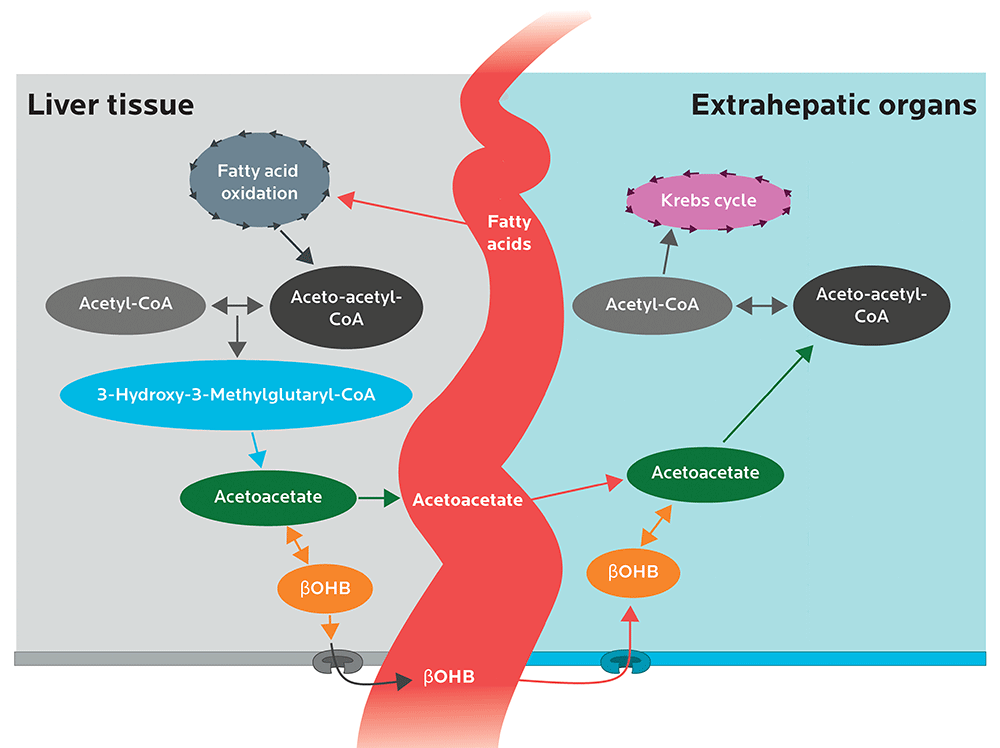
Image adapted from Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends in Endocrinology and Metabolism. 2014 Jan 31;25(1):42-52.
Mutations in the HMGCS2 gene are associated with a genetic condition known as HMG-CoA synthase deficiency.
When eating normally, people with HMG-CoA synthase deficiency are fine. But when fasting, they cannot go into ketosis properly (aka hypoketotic); they may become hypoglycemic enough to go into a coma.
HMG-CoA synthase deficiency isn’t a medical condition that needs treatment. Affected people simply need to avoid long periods of fasting… and probably also need to quit reading diet support groups.
If you’re worried you might have this, probably don’t worry too much: It’s a relatively rare, autosomal recessively inherited disorder (in other words, you need two copies of the mutant gene) that only affects fewer than 1 in 1,000,000 people.
Most of us won’t have this rare genetic disorder. Yet given the complexity of metabolism, it’s still quite possible that we may differ in our responses to a ketogenic diet.
What this means for you
- Genetic testing may tell you whether you have the HMGCS2 rs28937320 variant that could affect your response to a ketogenic diet and fasting. 23andMe doesn’t test for the variant, but other commercial services may. Again, this variant is quite rare, so there’s a strong chance you don’t have it.
- If you want to know whether your body will thrive on a ketogenic diet… try a ketogenic diet for a few weeks, and see what happens. A standard ketogenic diet protocol that is used to treat childhood epilepsy typically uses a 4:1 ratio of dietary fat to protein and carbohydrate combined (in other words, 4 grams of fat per 1 gram of combined protein and carbohydrate). Generally, it takes 2-3 days of eating this way to get into ketosis. If you try several weeks of keto dieting and feel terrific… or terrible… or “meh”… then you have your answer. The data you gather from this simple experiment will probably give you a clearer answer than most genetic predictions we could make right now.
- Experiment and discover the diet that is right for you. Other people’s results with a given diet may not match yours, in part due to possible genetic factors.
Micronutrients: Folate and MTHFR variations
The MTHFR gene codes for the enzyme methylenetetrahydrofolate reductase (MTHFR).
This enzyme helps us process Vitamin B9, or folate (specifically, folic acid), which occurs naturally in a wide variety of foods and is involved in many physiological processes, including having a healthy pregnancy.
Two common MTHFR polymorphisms (rs1801133 and rs1801131) affect how our bodies metabolize this nutrient. Approximately 60 to 70% of people will have one of these variations. About 10% of people will have both polymorphisms.
When MTHFR protein levels are lower (particularly when one has two copies of the rs1801133 polymorphism), we don’t process folic acid as well. Homocysteine levels may go up. Higher homocysteine is linked to inflammation and chronic diseases such as heart disease and stroke.
Table 10.1: Two common MTHFR polymorphisms and their effects
| rs1801133 (677 C>T) | rs1801131 (1298 A>C) | ||
| Allele | What it means | Allele | What it means |
| CC | Normal folate metabolism | AA | Normal folate metabolism |
| CT | 65% efficiency in processing folic acid | AC | Possibly impaired folate metabolism |
| TT | 10-20% efficiency in processing folic acid | CC | Number of risks. Complex. |
However, these more common MTHFR polymorphisms don’t cause severe MTHFR deficiency, which is a rarer, genetically recessive condition that can result in major neurological, movement, and psychiatric disorders as people age.
MTHFR, like most genes, doesn’t act alone.
For the MTHFR protein to do its job, it also needs riboflavin (aka vitamin B2) as a cofactor, a substance that’s required for an enzyme to work. Riboflavin is involved as a cofactor in a wide range of reactions and biological processes.
Thus, genes involved in the absorption, metabolism and utilization of riboflavin will influence the effect of whatever MTHFR gene variant one has.
Right now, we know of about 90 genes in the human genome that code for proteins that depend on riboflavin. There are six genes for riboflavin uptake and conversion to the active coenzymes flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), and two for converting the enzyme to another form, dihydroflavin.
This means that our ability to use MTHFR effectively may depend on many complex factors beyond having a genetic MTHFR variant.
While there are quite a few genetic testing services out there that offer advice on B vitamin supplementation based on genetic data, the U.S. Academy of Nutrition and Dietetics argues that currently, there isn’t enough evidence about MTHFR polymorphisms to change current folate recommendations.
Likewise, the American College of Medical Genetics and Genomics actively discourages testing for the two common polymorphisms in the MTHFR gene, arguing that:
- it has “minimal clinical utility”;
- there is no evidence that specific treatments lower the risks of high homocysteine or other health conditions linked to MTHFR variants; and
- concerned patients might be better off simply testing their homocysteine levels directly.
What we found in our sample
23andMe tests for a variety of MTHFR polymorphisms. Here’s what we found for the two most common risk variants.
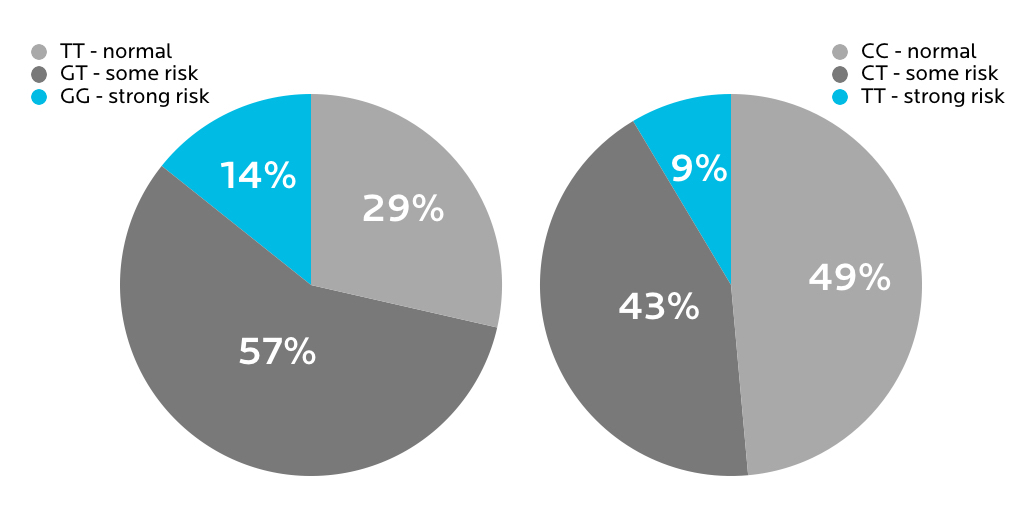
However, it wasn’t clear whether having any of these variants had actually led to any health problems for the affected people.
What this means for you
- As usual, it’s complicated. Genes, proteins, and nutrients interact in highly complex ways.
- If you have a MTHFR polymorphism, your body may not be as efficient as others’ at processing folate. This is especially important for women considering pregnancy. However, it’s worth exploring if you have other health concerns that have been definitively linked to low folate, such as anemia.
- Work with a qualified healthcare provider if you have concerns. You can get your homocysteine levels tested directly with a blood test. Don’t rely only on genetic testing data, which at best is simply a prediction of risk rather than a definitive measure.
Caffeine metabolism
You might have noticed conflicting reports in the media about whether caffeine is “good” or “bad” for you.
One problem in judging research studies on things like the link between, say, coffee and heart disease, is that we vary genetically in how we process caffeine.
The gene CYP1A2 codes for the cytochrome P450 “superfamily” of enzymes, involved in the breakdown of drugs along with cholesterol, sterols, and other lipids. Caffeine is mainly metabolized by the liver enzyme known as P450 1A2, and breaks down into byproducts like theophylline, paraxanthine, and theobromine (the compound in dark chocolate that is supposed to make us feel good).
Variations in the CYP1A2 gene at the rs762551 SNP determine how quickly a person will metabolize caffeine.
- If you have the “slow” version, more caffeine seems to raise your chronic disease risk.
- If you have the “fast” version, more caffeine seems to lower your chronic disease risk.
However, this is just about how your body processes caffeine.
It doesn’t mean that your caffeine consumption habits are determined by your genes, though there may be some genetic basis for that as well.
Studies comparing genetically identical twins suggest that inheritance may explain between 34-58% of caffeine-related effects and consumption patterns… unless you’re a heavy caffeine user, in which case the contribution of inheritance went up to around 77%.
For people who were light or moderate caffeine consumers, inheritance seemed to matter less than environment — for instance, what people around them were doing, whether they liked the taste of coffee or tea (for more on food preferences, see Chapter 8), etc. But for people who were heavy caffeine consumers, heredity seemed to play a bigger role… though still not 100%.
What we found in our sample
Many of us at PN are big coffee and tea fans — so much so that having high-end coffees and teas is a much-coveted part of PN gatherings. People roast their own beans, argue over the best coffee roast or place in a given city, and would never be caught drinking cheap green tea.
You’d think that given this love for the magical alkaloid caffeine, all of us might be fast metabolizers.
In fact, only half of us are.
Would we change if we knew our caffeine processing type?
It’s hard to say, but…
If we’re being honest… probably not.
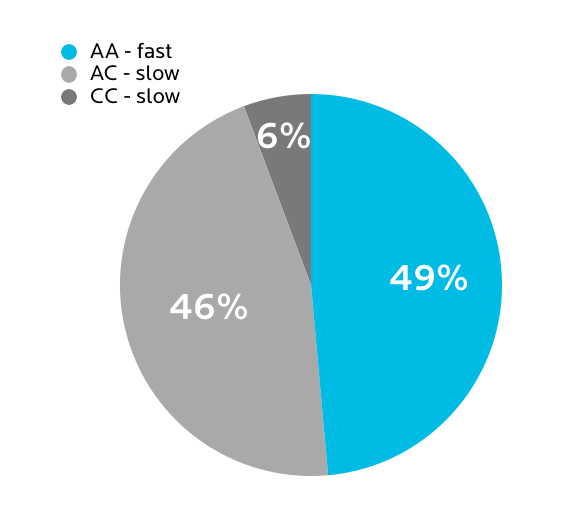
What this means for you
- This one might be worth getting tested for… but only if you’ll actually do something about it. If you have the “slow” version but won’t give up your daily pot of coffee, well… you might as well save your testing money and spend it on espresso instead.
What’s up next
In the next chapter, we’ll look at how genetic variation might affect our athletic performance and response to exercise.





