Our gastrointestinal tracts work hard to keep us healthy and happy. When gut health is compromised, we can face major health consequences. Here’s how to use good nutrition to keep your digestion humming along.
“Heal the gut and you heal yourself.” – Gerard E. Mullin, MD
At least 70 million people in the U.S. suffer from some sort of digestive illness (not including heartburn), and digestive problems account for nearly 10% of all healthcare spending.
The hard-working gut allows nutrients and water to enter the body while preventing the entry of toxins/antigens. It’s a selective barrier between “us” and the outside world. But a distressed gut can’t act in our defense. Instead, it allows dangerous compounds to enter the body.
That’s where nutrition can come in. The right diet strengthens the gut in its guardian role, improving overall health and well-being.
What can go wrong
If Mama ain’t happy, ain’t nobody happy. Substitute “gut” for “mama” and you pretty much get the picture.
If your gut is distressed, it won’t perform well and you won’t feel good.
A trip to your doctor might end with a diagnosis of irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), leaky gut (LG), celiac disease, food sensitivities, bacterial imbalances – or no specific diagnosis at all, since symptoms often overlap and it can be tricky to untangle the root causes of digestive disorders. For more on diagnostic criteria, see MayoClinic.org.
What’s unquestionable is that a healthy gut barrier depends on:
- balanced intestinal bacteria (our gut contains about 3-4 pounds of bacteria);
- intact mucosa (our gut lining replaces itself every 3-7 days); and
- a healthy immune system (almost 70% of our immune system cells live in or around the gut).
If any of these are unstable, your gut won’t be happy – and neither will you.
Our gut bacteria: Hard-working cells
Factoid: You have more bacteria in your gut than cells in your body!
Bacteria can be classed as harmful or helpful. Beneficial bacteria are like busy tourists in our guts. They come and go. We don’t have a permanent supply, so for a vibrant gut “economy,” we need to continually replenish them via diet.
Our gut bacteria vary depending on age, gender, diet, geography, hygiene, stress and medication use. Birthing method (C-section vs. vaginal delivery) and first foods (breast milk vs. formula) can also determine what bacteria colonize our gut, with breast milk being an “immunological asset,” because it generally increases the number of friendly bacteria.
Beneficial gut bacteria help manufacture vitamins (B12, K, B6, B5, B3, folate and biotin), enhance absorption of minerals, fight off pathogens, digest food, and metabolize drugs. They even influence total body metabolism!
Balancing beneficial bacteria
Using antibiotics can eliminate beneficial bacteria in our gut, creating a prime environment for yeast (Candida albicans) growth. Candida, in turn, can provoke inflammation and symptoms associated with IBS. (However, as contradictory as this may seem, antibiotics are sometimes used to treat IBS symptoms.)
Reduced beneficial bacteria can also occur with low iron levels and/or a low carbohydrate diet. At the same time, excessive carbohydrate consumption can contribute to small intestinal bacterial overgrowth (the bad kind), aka SIBO. So for folks who suffer from SIBO, a limited carbohydrate and higher protein/fat diet can be useful.
Eating enough fibre may play a significant role in gut health. Fibre resists digestion in the small intestine, then makes its way to the large intestine and ferments, creating short chain fatty acids, an important source of fuel for the body.
Fibre also adds bulk and improves regularity, reducing our exposure to potentially dangerous compounds. Finally, the breakdown of fibre regulates pH balance, promoting the optimal environment for beneficial bacteria.
Intact mucosa & healthy immune system
Much of what we consume today was unknown to our bodies just 100 years ago. Some experts speculate that the introduction of these new compounds explains the increase in food intolerance and allergies. Our gut simply can’t handle them!
When the gut wall is irritated or inflamed, the tight junctions between its cells loosen up and we get increased permeability (or leaky gut syndrome). Inflammation, stress, pharmaceuticals, bacterial balance, malnutrition, compounds in food (gluten, casein, lectins, fructose, etc), and food additives (including MSG) can all influence the junctions in our gut and weaken their bonds.
A leaky gut isn’t very selective. It might slam the door on beneficial nutrients while welcoming dangerous bacteria inside. This is called bacterial translocation, or BT. It can stimulate an immune response or inflammation, and it burdens the brain and liver.
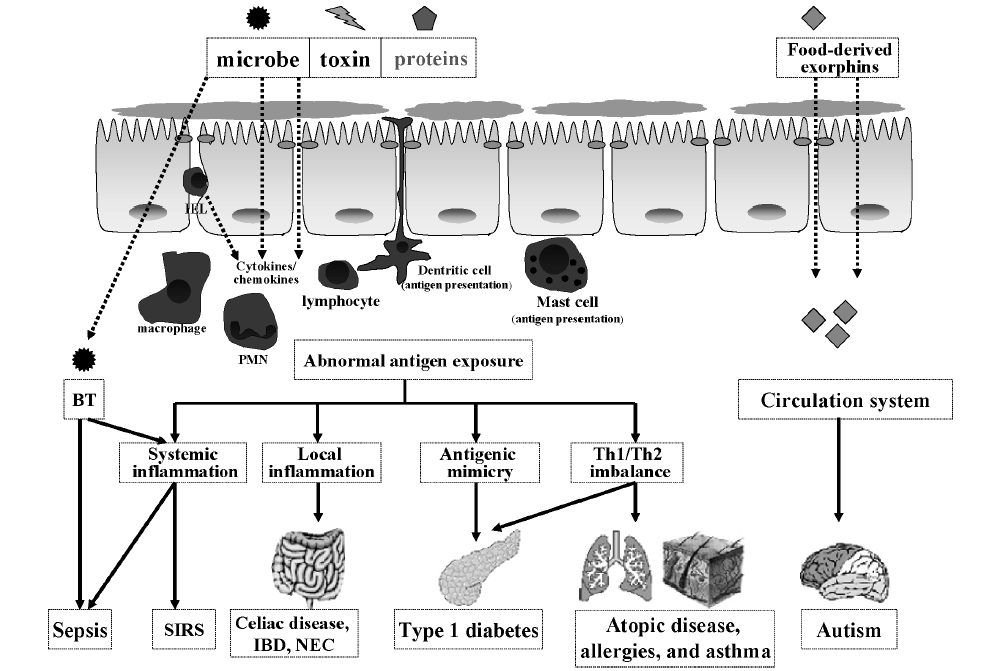
A leaky gut often goes along with conditions such as:
- autism;
- Type 1 diabetes;
- allergies;
- mental illnesses (including depression and schizophrenia);
- skin inflammation such as acne, rosacea, and eczema;
- diminished insulin signaling; and
- asthma.
Although causal relationships aren’t established, researchers hypothesize that certain compounds (e.g., gluten, casein) cross the leaky gut and provoke an antigenic response, leading to central nervous system dysfunctions.
What causes a leaky gut?
Contributors include:
- the long term use of pharmaceuticals (most notably non-steroidal anti-inflammatory drugs [NSAIDs], birth control, and corticosteroids);
- excessive sugar/refined carbohydrate consumption;
- excessive alcohol consumption (although red wine in moderation seems to improve gut health);
- pathogenic bacteria (e.g. infections from H. pylori and E. coli), which can compromise gut health for up to three years;
- parasites, yeast, stress (acute & chronic); and
- environmental contaminants.
Gut feelings
Our gut communicates with all cells in the body, which means that disturbances in the gut can show up as disturbances in the brain (and vice versa). As a matter of fact, the brain actually kicks off digestion before the gut — we secrete acids and digestive enzymes before even swallowing the first bite of a meal!
In addition, our emotions influence gut health.
When you’re afraid, your brain and gut know, and your digestion slows down. Ever had the experience of not being able to eat when you’re feeling especially anxious? That’s because blood flow and enzyme production in the gut are limited during stress.
At rest, the gut receives over half of all organ blood flow, but during exercise, blood flow to the gut can drop to less than 20% of this resting value. Lack of blood flow to the gut during digestion can lead to increased intestinal permeability.
Ironically, both endurance exercisers and people with heart failure are susceptible to leaky gut syndrome; in each case (though for entirely different reasons) not enough blood is making it to the gut.
It’s important to note that symptoms of a disturbed gut can show up outside the gut itself, manifesting as seemingly unrelated symptoms such as:
- joint pain;
- fibromyalgia;
- sleep disturbances;
- rheumatoid arthritis;
- fever;
- restless leg syndrome;
- anemia;
- skin irritation;
- fatigue;
- night sweats;
- headache and so on.
Serious gut pathologies often result in weight loss and nutrient deficiencies (since malabsorption is occurring).
What causes gut distress?
Often, it’s the foods we eat. Foods that are healthful for some people might not be healthful for you. Four common offenders:
- Lectins: particular types of proteins. The most irritating type is found in seeds such as grains, beans/legumes, and nuts.
- Gluten and other similar prolamine proteins (such as hordein in barley, secalin in rye, or zein in corn), found in grains.
- Casein, lactose, and other immunoglobulins in dairy.
- Fructose, aka fruit sugar. People who struggle to digest fructose also often have trouble with other complex carbohydrates known as FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides and polyols). More information on a low FODMAP diet
For some, these compounds can induce mast cells to produce histamine, mimicking a food allergy and increasing intestinal permeability and inflammation. Or they can mimic symptoms of respiratory allergies, such as sneezing, sniffles, and throat irritation.
For others, these foods stimulate an immune system T-cell response and create or exacerbate autoimmune symptoms such as joint pain or skin rashes (particularly eczema).
Other people simply lack the appropriate digestive enzymes to process one or more of these compounds. In this case, you might just get a general stomach upset, gas and bloating, nausea, and constipation or diarrhea.
Interestingly, some of the foods that contain these compounds can have addictive properties, creating an immediate feeling of well-being. So, while your gut might not be suited to digest casein, right after you drink milk you get a rush of “feel good time”, only to soon be reminded of the gut upset that follows.
For more, see here:
How to improve your gut health
“The intestinal barrier operates as the biological door to inflammation, autoimmunity and cancer.” – Allesio Fasano, MD
Get to the root cause. While there can be many causes of gut troubles, there is always a cause. Identify it before you mask symptoms with medications.
Eliminate any foods/drinks you know to be problematic. Do this on your own (see here: DigestiveWellnessBook.com and How To Do an Elimination Diet) or set up an elimination diet with a professional.
Balance your bacteria. Beneficial bacteria strengthen the intestinal barrier. Choose 1-2 probiotic/prebiotic rich foods/drinks and consume them regularly. See here for ideas: All About Probiotics
Eat when hungry, stop when satisfied. If someone is having gut problems (and still gaining body fat), the first place to look is overconsumption of sugars, processed grains, processed meats, dairy, and rich meals.
Sugar alcohols can wreak havoc in the gut. If you are struggling with bloating and cramping, eliminating sugar alcohols might be a wise place to start (think sugar free desserts, gum, protein powders, protein bars, etc).
Slow down. The process of slowing down and chewing is important for enzyme release and breaking food down into particles that are manageable for the gut.
Consider glutamine. Glutamine can help reverse excessive intestinal permeability, act as fuel for intestinal cells, and might attenuate the allergic response.
Consider digestive enzyme supplements. Look for a broad-based multi-enzyme formula. Many of us produce less hydrochloric acid — a key digestive component of our stomachs — as we age; look for a formula that includes betaine HCl. (However, if this type of formula gives you heartburn, stick to the regular enzyme supplements without betaine.)
Check vitamin D levels. Low vitamin D status might decrease immune function and is associated with IBD.
Check iron levels. Decreased iron status is associated with poor gut function. This can result from gut malabsorption with the consumption of mineral-binding foods such as grains and legumes, or simply a low iron intake. Vegetarians/vegans and endurance athletes are especially prone to this.
Supplement wisely. Natural compounds that might help gut health include St. John’s Wort, melatonin, curcumin (turmeric), Iberis amara, chamomile, arrowroot, peppermint, Boswellia carterii, artichoke leaf, clove, zinc, quercetin, gamma oryzanol, licorice root, CoQ10, phosphatidylcholine, aloe vera and psyllium. But ideally, solve the underlying problem (e.g. digestive intolerance) first.
Eat plenty of omega-3s (flax, walnuts, hemp, chia, fish, algae) and other whole food fats (olives, avocado, coconut, nuts, seeds, etc) to help moderate inflammation. Also note that medium chain fats, found in coconut, can also help with gut health.
Flavonoids (this includes isoflavones, anthocyanidins, flavones, flavonols, flavan-3-ols and flavonones) can help improve gut health. Fruits, vegetables, beans (including soy), tea and coffee are the major sources of flavonoids in the human diet. Foods in the cabbage family and vegetable broths can also help here. On the other hand, if FODMAPs are a problem for you, choose carefully, as some of these foods may cause more trouble.
Recover well. Sleep, stress management (e.g., meditation, yoga) and exercise are necessary for renewal of the body and controlling inflammation. Improving these areas may improve gut health. Remember that excessive exercise can lead to poor gut health. Avoid big meals before exercise.
Eat real food. Our bodies have a longstanding relationship with whole/real foods. Food preservatives and additives, on the other hand, present a new (and perhaps impossible) challenge for our bodies.
Get fibre. Nutrient-dense, high-fibre carbohydrates like vegetables are important in the diet. Eat your veggies! And if you’re like most Westerners, you probably need more fibre. Try beans, peas, vegetables, nuts, seeds, fruits and whole grains. For more see here: All About Fibre
Breast-feed. Children who are breast-fed tend to have less gastrointestinal infections and inflammatory disorders.
Avoid common triggers such as:
- added sugars;
- refined grains;
- MSG (see here: Hidden Sources of MSG);
- NSAIDs (such as ibuprofen or naproxen drugs);
- acid blockers; and
- alcohol (except red wine in moderate amounts).
These harm our healthy bacteria, disrupt the delicate chemical ecosystem of our GI tracts, and/or cause additional gut damage (e.g. NSAIDs can cause GI bleeding).
Reduce your chemical burden. Choose organic when possible, avoid heating foods in plastics, use clean body products, avoid food colorings/preservatives and avoid fish high in toxins.
When ya gotta go, ya gotta go. If you need to evacuate your colon, do it. Avoid waiting. One to three bowel movements per day = good.
Extra credit
If the vagus nerve (which connects brain to gut) is cut, the gut functions fine on its own.
You have more nerve cells in your bowel than in your spine.
80-90% of serotonin is made in the gut.
AGEs may enter the body more readily if someone has a LG. For more see here: All About Cooking and Carcinogens
Gerd Gigerenzer (German psychologist) said the enteric nervous system is the intelligence of the unconscious.
References
Click here to view the information sources referenced in this article.
Eat, move, and live… better.
Yep, we know… the health and fitness world can sometimes be a confusing place. But it doesn’t have to be.
Let us help you make sense of it all with this free special report.
In it you’ll learn the best eating, exercise, and lifestyle strategies — unique and personal — for you.



Share