Cabbage: not just for coleslaw any more! Learn about pH strips and make your own, right in your own kitchen.
pH strips are pieces of paper that change color depending on the pH – the acidity or alkalinity – of a liquid. pH strips are a cheap and relatively accurate way of measuring the pH of any liquid, in our case urine. A strip of filter paper is soaked with different pH indicators (more on that later), allowed to dry and voila: pH strips. Most common pH strips are designed to test urine, water and saliva.
There are a variety of pH strips available. They differ by sensitivity and what range of pH they are designed for. The more sensitive the pH strip, the smaller the range in which it works.
Why the limitation? Well, you can only differentiate so many colours. Figuring out whether your strip is bluish-green or greenish-blue can be difficult.
Now, depending on what pH range you want and how sensitive you need the strip to be, you adjust your indicators. So you can have a pH strip with a range of 1-14, but not very sensitive; or you can have a pH strip with a range of 5.5-8.5 that can measure changes as small as 0.25 in pH.
The next question is how do you know what pH is bluish-green? To make a colour legend (i.e., to figure out what colour goes with what pH) the pH strips are dipped into solutions with different known pHs. If you dip your pH strip into a solution with a pH of 5 and the strip is dark blue then you know that blue means pH of 5, and so on until you cover the range of the strips. You don’t have to do this part; someone’s probably already done it for you — the colour legend will be included with the package of strips.
pH indicators
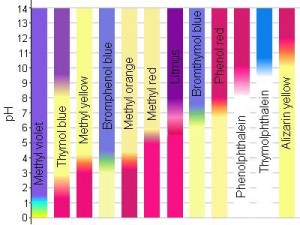
pH indicators are usually weak acids or weak bases that change colour at specific pHs. For instance, methyl red is a common indicator that’s red at pH of 5 and yellow at a pH of 6.
Super-mini-chem lecture
Why do they change colour? I’ll explain the theory very briefly. There are a few different ways of defining acids and bases, but I think the easiest way to understand them is to think of them as chemicals that exchange a proton. In this case, the proton comes from a hydrogen ion – a hydrogen with a positive charge, which gives us the “H” in “pH”. Chemicals that donate a proton are acids; chemicals that take up a proton are bases (aka alkaline). So hydrogen donor – acid; hydrogen acceptor – base.
Colour changes occur when the acid or base accepts or donates a proton. When this happens depends on the chemical’s specific characteristics. Some indicators are more complex and have more than one colour change. See our experiment below for an indicator with multiple changes: first from red to blue, then from blue to yellow.
Luckily, there are a wide range of pH indicators that change at a bunch of different pHs as well as change to a bunch of different colours, so that specific pH strips can be made.
Home experiment
If you’re interested you can make your own pH strip with a red cabbage, some boiling water and a McGyver wig (optional).
First, purée your red cabbage with 2 cups of boiling water (and you thought your blender was only good for shakes!). Next, strain the bits with a fine mesh strainer and keep the liquid. (You can make cabbage soup with the leftovers.) The liquid is your pH indicator (or cabbage tea — call it what you will).
Get a coffee filter, cut it in strips and soak the strips in the cabbage tea – I mean pH indicator – for a few hours. Take the strips out and let them dry. Make sure not to drip any, as it will stain. Ta da! Your very own pH strips!
Your pH strips will most likely be violet-bluish (pH 7) to start (see below). Try dipping your strips into vinegar, lemon juice, cola, baking soda solution, antacids or bleach (be very careful) and watch it change colour. Really, the list is endless – though you might not see much of a difference with some. Use a different strip each time for best results. Have fun!
| pH | 2 | 4 | 6 | 8 | 10 | 12 |
| Colour | Red-orangish | Purplish | Violet | Blue | Blue-green | Greenish yellow |
Learn more
Want to get in the best shape of your life, and stay that way for good? Check out the following 5-day body transformation courses.
The best part? They're totally free.
To check out the free courses, just click one of the links below.




Share